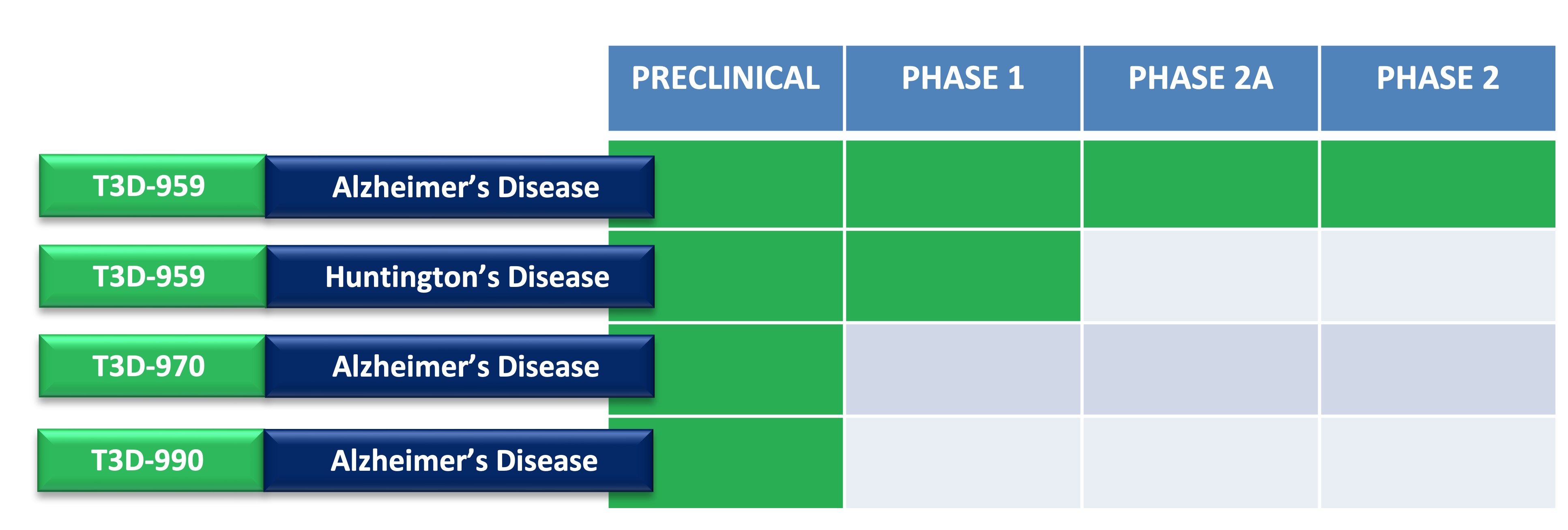
Pipeline
T3D Therapeutics has an exclusive, worldwide license for T3D-959 and structurally-related small molecules. Their mechanism of action may provide therapeutic benefit in neurodegenerative diseases such as Alzheimer’s Disease (AD) and Huntington’s Disease (HD).
Lead compound T3D-959 is in active development as a potentially disease-modifying, orally-delivered, once-a-day medicine for Alzheimer’s disease patients with mild to moderate disease severity and potentially for patients with mild cognitive impairment (MCI).